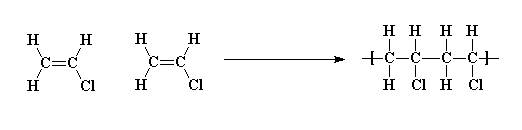Polyvinyl chloride c2h3cl n references functional groups vinyl ethenyl group c h bond is nonpolar van der waals dipole dipole induced dipole very strong and rigid references from scientific journals thank you for watching ameer a.
Poly vinyl chloride dipole.
The most important forces in pvc are dipole induced dipole attractions.
Polyvinyl chloride is produced in an addition polymerisation reaction using the chloroethene vinyl chloride monomer.
Vinyl chloride h2c chcl or c2h3cl n or c2h3cl cid 6338 structure chemical names physical and chemical properties classification patents literature biological activities safety hazards toxicity information supplier lists and more.
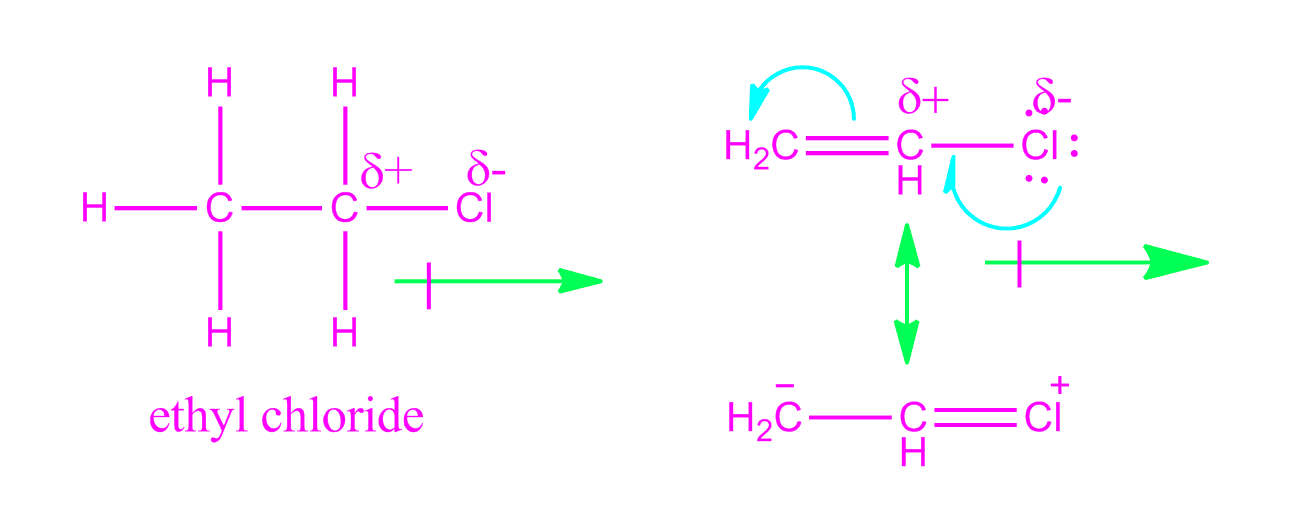
It is tempting to say that the molecules line up as in the diagram below.
Polyvinyl chloride is a white rigid quite brittle solid.
Cruz et al.
Pvc vinyl chloride is an organohalogen compound that has important industrial applications.
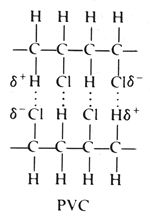
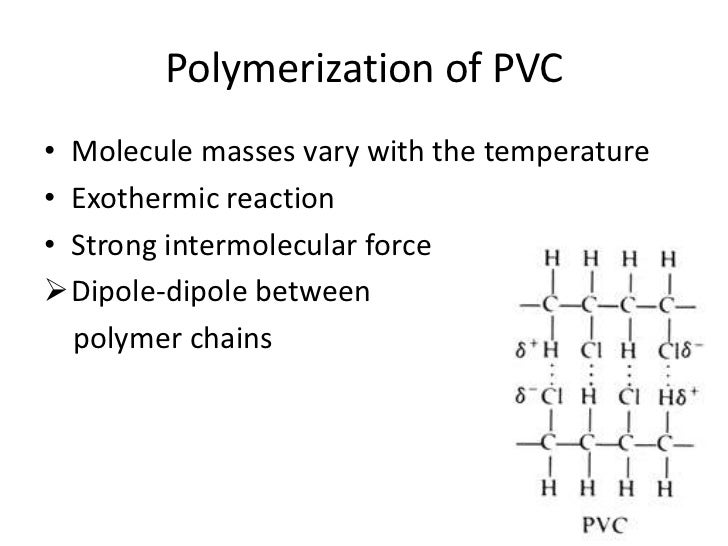
Pvc consists of polar molecules which are attracted to each other by dipole dipole interactions due to electrostatic attractions of a chlorine atom in one molecule to a hydrogen atom in another atom.
Additives are used to modify the properties of polyvinyl chloride to make it more useful.
Pvc comes in two basic forms.
This polymerisation reaction proceeds by a free radical mechanism.
Pvc is the world s third most widely produced synthetic plastic polymer after polyethylene and polypropylene.
For instance eknoian and his coworkers prepared copolymers of oxazolidinone monomers with acrylonitrile polyvinyl chloride polyvinyl alcohol polyvinyl acetate and styrene through an emulsion.
Pvc is a polymer of vinyl chloride.
About 40 million tons of pvc are produced each year.
Pvc is used in the manufacture of numerous products including packaging films and water pipes.
When treated with certain catalysts vinyl chloride monomers undergo polymerization and form the larger compound known as polyvinyl chloride or pvc.
Polyvinyl chloride economical versatile polyvinyl chloride pvc or vinyl is used in a variety of applications in the building and construction health care electronics automobile and other sectors in products ranging from piping and siding blood bags and tubing to wire and cable insulation windshield system components and more.
It looks as if are dipole dipole forces between adjacent molecules but the cl atoms are large and they stick out from the chain in random directions.
Rigid sometimes abbreviated as rpvc and flexible.
These considerable intermolecular attractions between polymer chains make pvc a fairly strong material.