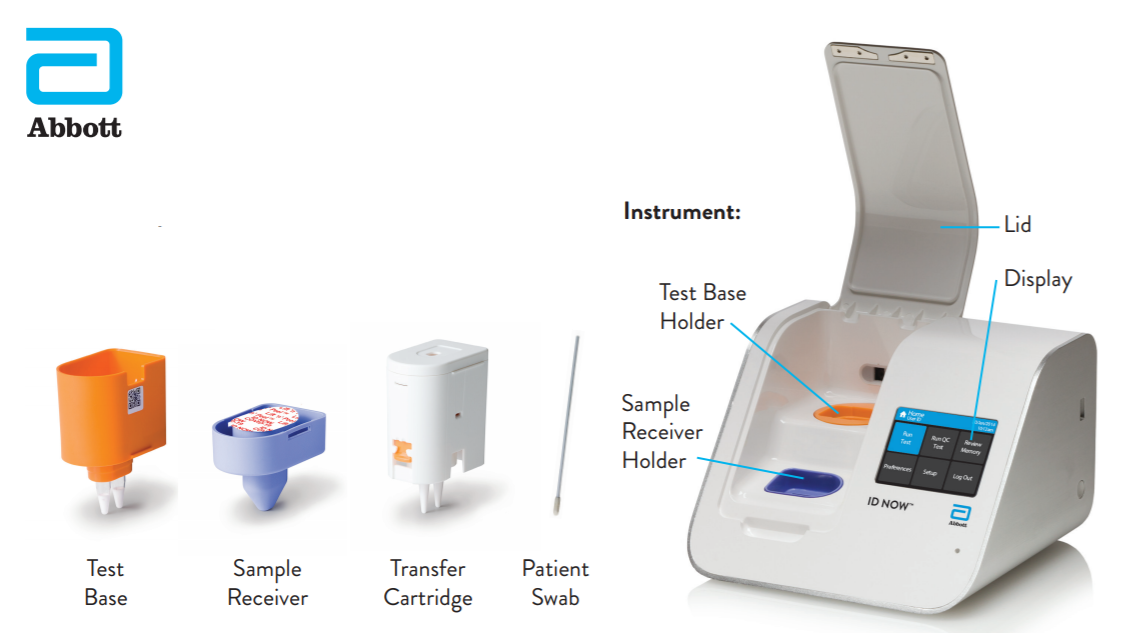
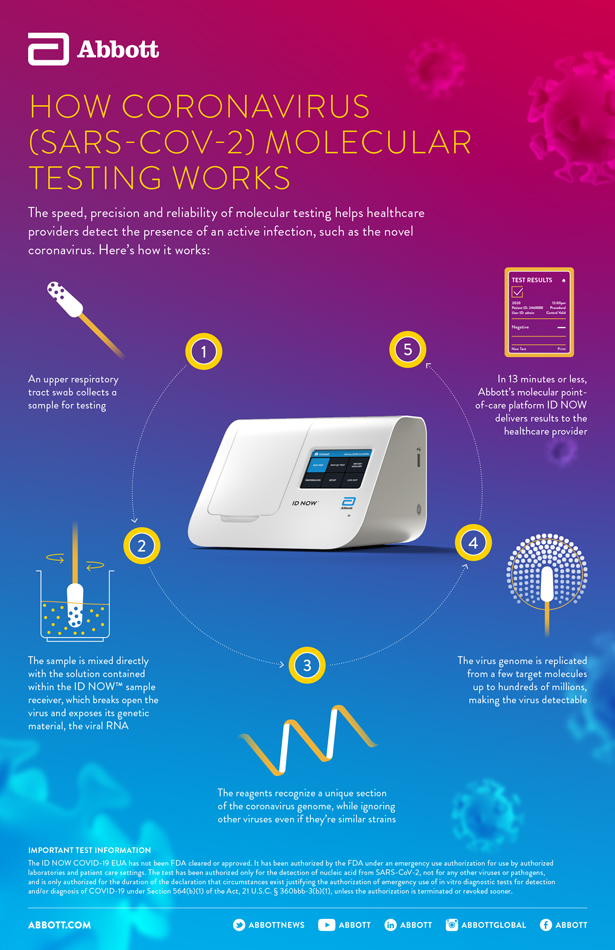
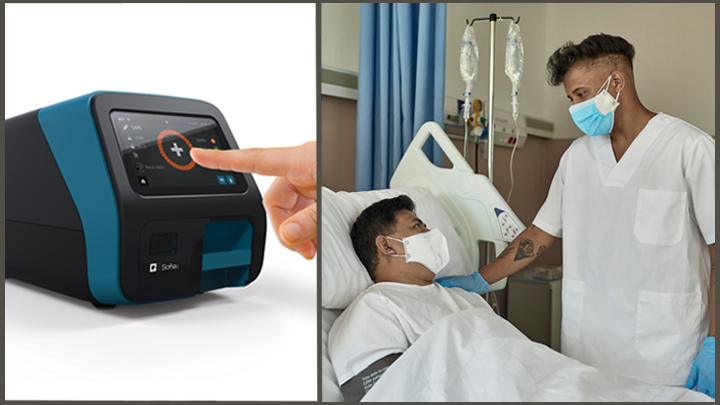
This test is performed at the same location as where the patient s sample is collected and it can provide covid 19 results in under 13 minutes.
Point of care testing devices for covid 19.
Covid 19 rapid point of care test distribution the u s.
Point of care antigen test devices on july 14 centers for medicare and medicaid cms announced an initiative to distribute rapid point of care poc antigen covid 19 testing devices to nursing homes across the country.
Food and drug administration emergency use authorization eua.
Rapid point of care testing is a critical element of the national strategy for testing especially to support vulnerable patients outbreak investigations and frontline healthcare workers.
Nursing facilities will receive one of two testing devices.
Molecular point of care testing for covid 19 offers healthcare workers rapid results in more settings where people show up for care.
Food and drug administration emergency use authorization eua.
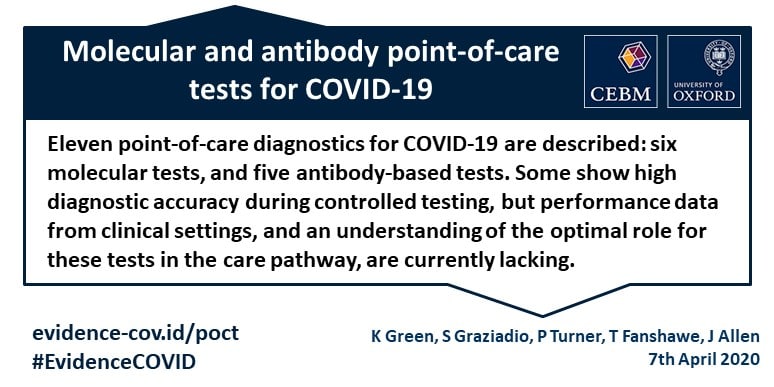
Six molecular tests and five antibody based tests.
The id now covid 19 rapid test delivers high quality molecular positive results in as little as 5 minutes targeting the coronavirus covid 19 rdrp gene.
Press release point of care testing poct devices market analysis 2020 covid 19 impact and global analysis by key companies comprehensive analysis by global industry share size growth.
The id now covid 19 assay is now available for use on the id now platform under u s.
Some devices show high diagnostic accuracy during controlled testing but performance data from clinical settings and a clear understanding of the optimal population and role for these tests in the care pathway are currently lacking.
The id now covid 19 assay is now available for use on the id now platform under u s.
Molecular testing technologies help detect the presence of a virus by identifying a small section of the virus genome then amplifying that portion until there s enough for detection.
Eleven diagnostic tests that are potentially suitable for testing for covid 19 at the point of care are described.
Every single nursing home across the country will be given point of care covid 19 tests by the administration starting next week officials announced tuesday.